SOLVED: How much will the temperature of a cup (180 g) of coffee at 95 ^ C be reduced when a 45 g silver spoon (specific heat 0.24 J / g^∘C )
By A Mystery Man Writer

VIDEO ANSWER: We are asked how much the temperature of a cup of coffee it is at 95 degrees celsius and we put in there a 45 gram silver spoon. The heat capacity is 0.24 joules per gram, degrees c. So here's my mass here's, my c and my initial
How much will the temperature of a cup (180 g) of coffee at 95 ^ C be reduced when a 45 g silver spoon (specific heat 0.24 J / g^∘C ) at 25^∘C is placed in the coffee and the two are allowed to reach the same temperature? Assume that the coffee has the same density and specific heat as water.
Numerade is a venture-backed, high-growth education technology startup based in Pasadena. We are singularly focused on creating exceptional video and interactive content experiences for education making the knowledge and skills of world class educators widely accessible and affordable to student audiences of all backgrounds. Our mission is to close the educational opportunity gap by unlocking and democratizing access to extraordinary educators and the content they have to offer.

⏩SOLVED:A 45-g aluminum spoon (specific heat 0.88 J/g ^ C) at 24 ^ C…
Solved] A cup contains 197 g of coffee at 98.3 °C. Suppose 60.3 g

8.2: Calorimetry (Problems) - Chemistry LibreTexts

Solved a) A student heats 60.45 grams of silver to 98.36°C
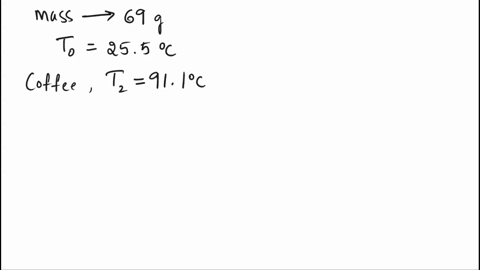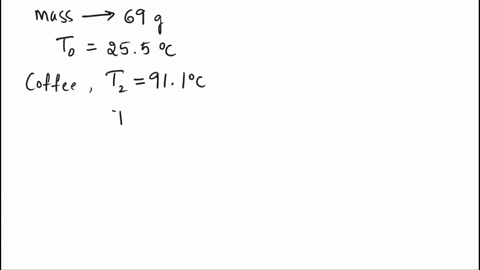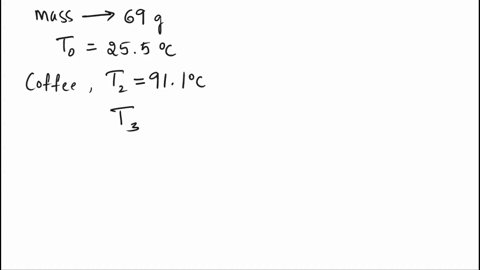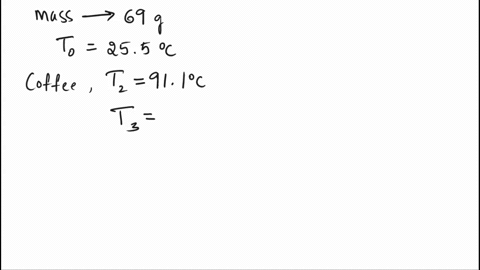
SOLVED: A 69.0 g silver spoon at 25.5°C is placed in a cup of coffee at 91.1°C. How much heat does the spoon absorb from the coffee to reach a temperature of

SOLVED: A 45-g aluminum spoon (specific heat 0.88 J/g°C) at 24 °C is placed in 180 mL (180 g) of coffee at 85 °C and the temperature of the two become equal.

Solved 3. How much will the temperature of a cup (180 g) of

Specific Heat Capacity

Chemistry 5.2 Flashcards

Answered: 2) An unknown piece of metal weighing…
Solved] 20. A 45-g aluminum spoon (specific heat 0.88 J/g °C) at 24 °C is
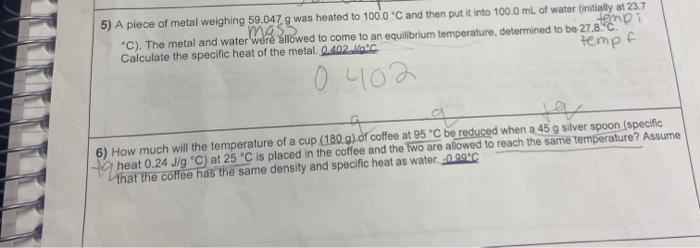
Solved temperalure of the two become equal. a) What is the