The compression factor (compressibility factor) one mole of a van der Waals gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is
By A Mystery Man Writer

Click here:point_up_2:to get an answer to your question :writing_hand:the compression factor compressibility factor for onemole of a van der waals gas at 0c
Click here👆to get an answer to your question ✍️ The compression factor -compressibility factor- one mole of a van der Waals gas 0-C and 100 atm pressure is found to be 0-5- Assuming that the volume of a gas molecule is negligible- calculate the van der Waals- constant a

The compression factor (compressibility factor) for one mole of a Van der..

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Sheet - 01 - Real Gas, PDF, Gases

The compression factor (compressibility factor) for one mole of a v

The compression factor (compressibility factor) one mole of a van der Waals gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is

6.3: Van der Waals and Other Gases - Physics LibreTexts

The compression factor (compressibility factor) one mole of a van der Waals'gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is negligible
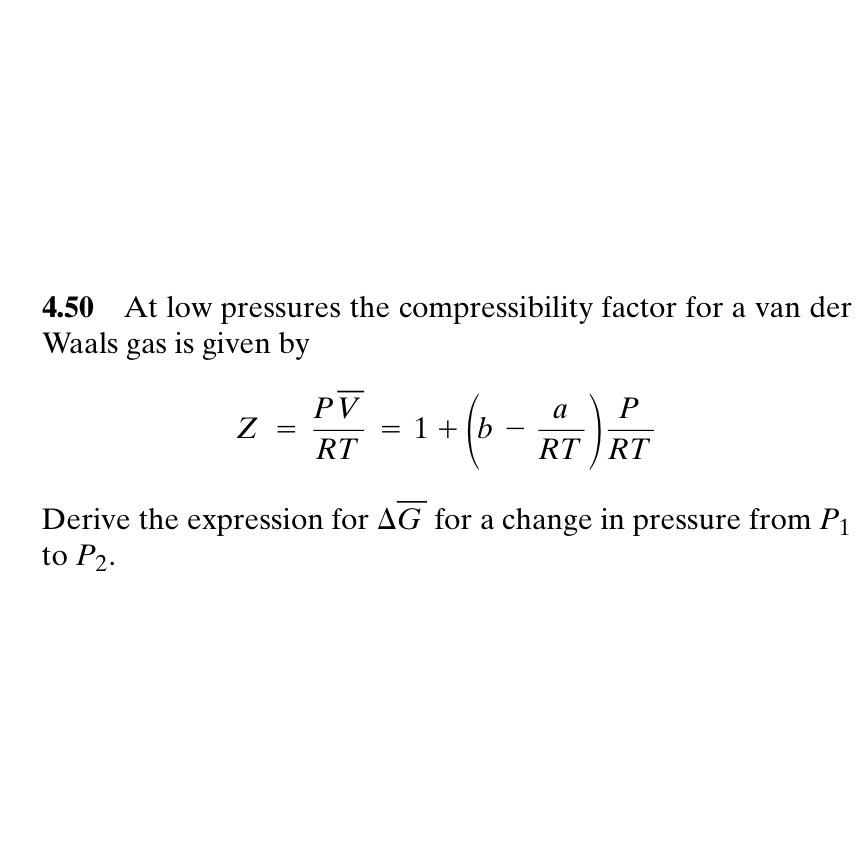
Solved 4.50 At low pressures the compressibility factor for

The compression factor (compressibility factor) for one mole of a Van der..

Djective lype Questions The compressibility factor of N2 moderate pressure range is equal to [where a & b are the van der Waal's constants] Pb Pb (2) 1- (1)RT RTV (3) (1RTV
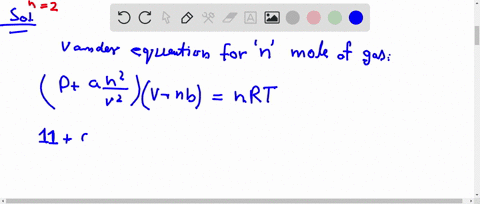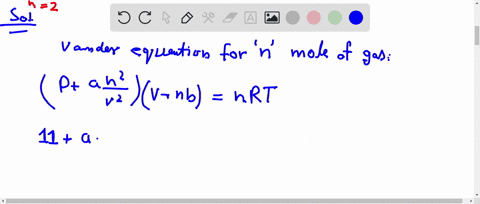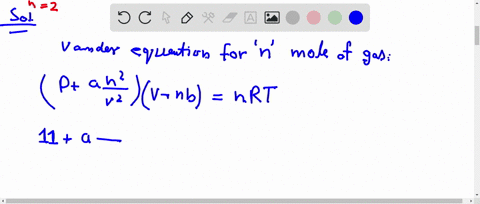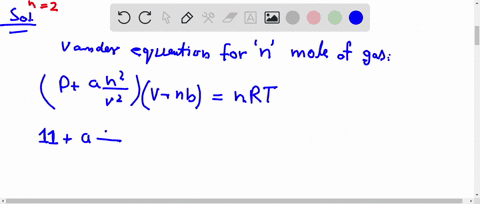
⏩SOLVED:The compression factor (compressibility factor) for one mole…

A gas has a compressibility factor of 0.5 and a molar volume of 0.4 dm3 mol− 1 at temperature of 800K