NEWS
At high pressure, the compressibility factor 'Z' is equal toa)unityb) c) d)ZeroCorrect answer is option 'C'. Can you explain this answer? - EduRev NEET Question
By A Mystery Man Writer


Solved 3.91. The definition of compressibility factor Z, Eq.

At a high pressure, the compressibility factor (Z) of a real gas is us

EGR 334 Thermodynamics Chapter 3: Section ppt video online download

Solved True or False? The compressibility factor of any gas
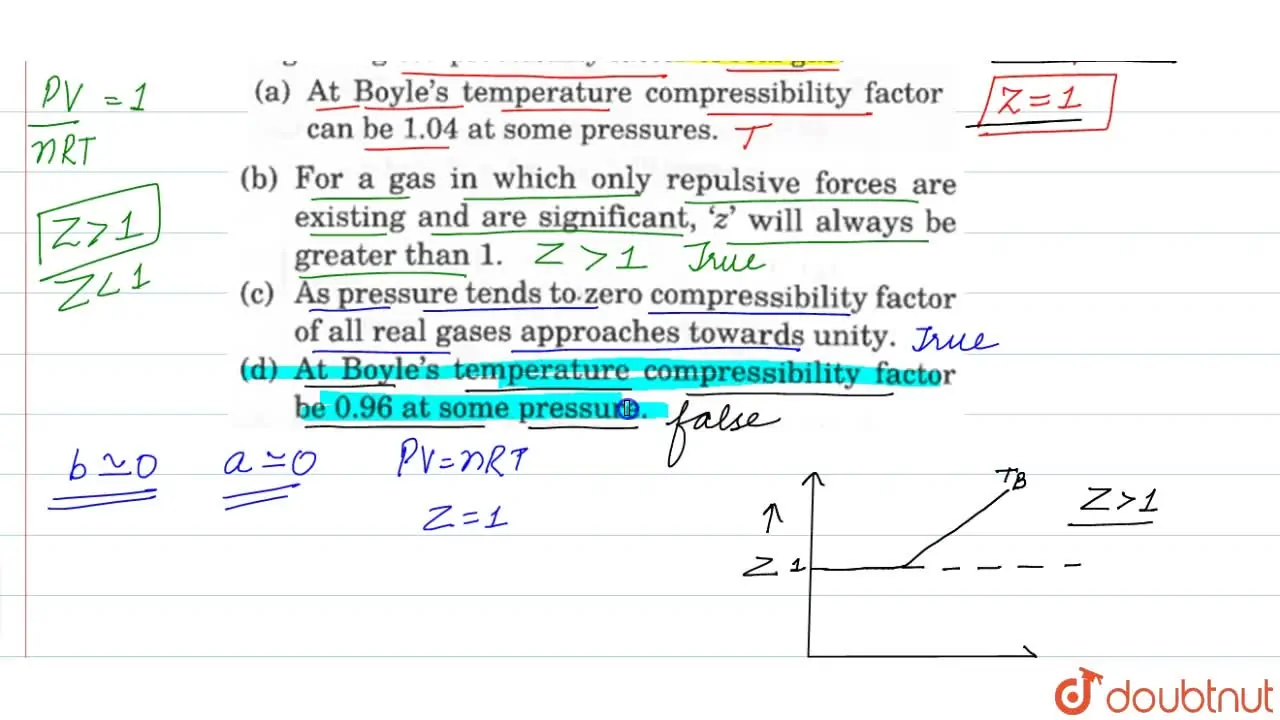
As pressure tends to zero compressibility factor of all real gases app
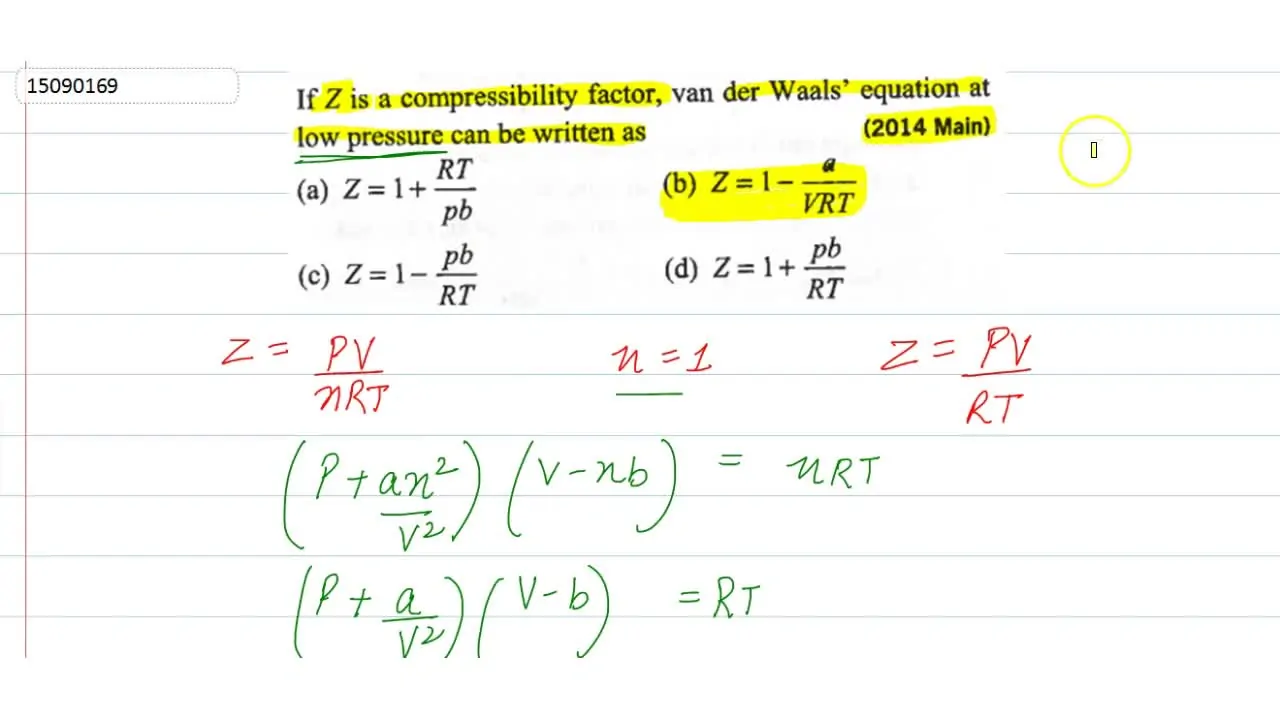
If Z is a compressibility factor, van der Waals' equation at low press

Why compressibility factor of areal gas is greater than unity at high pressure and temperature? - Quora
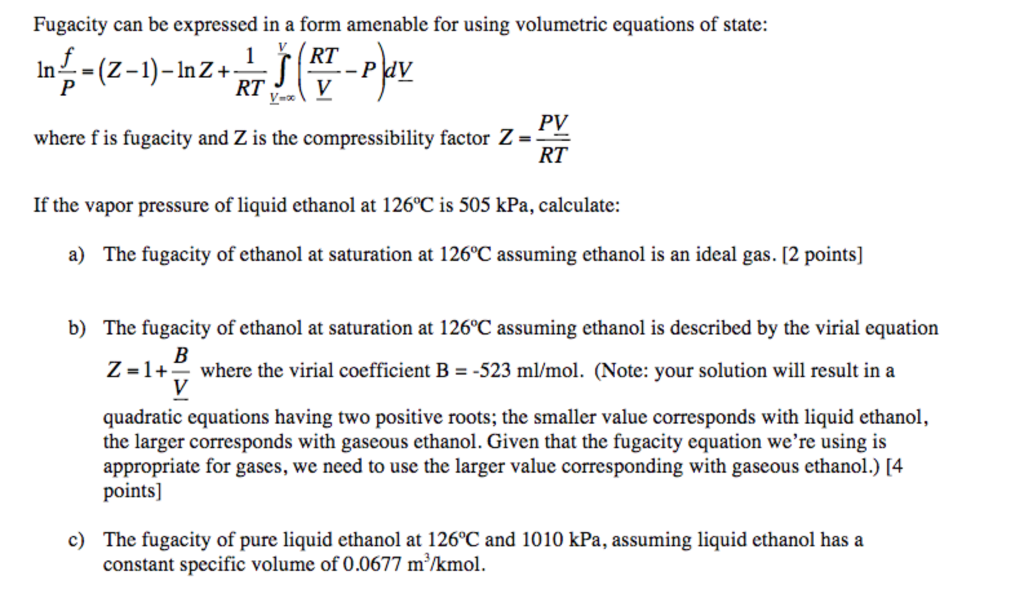
Solved Fugacity can be expressed in a form amenable for
The value of compression factor at the critical state of a vander waals gas is

Solved 2. (20 points) Use the generalized compressibility