
The state of an ideal gas is changed through an isothermal process at temperature T 0 as shown in figure. The work done by the gas going from state B to C
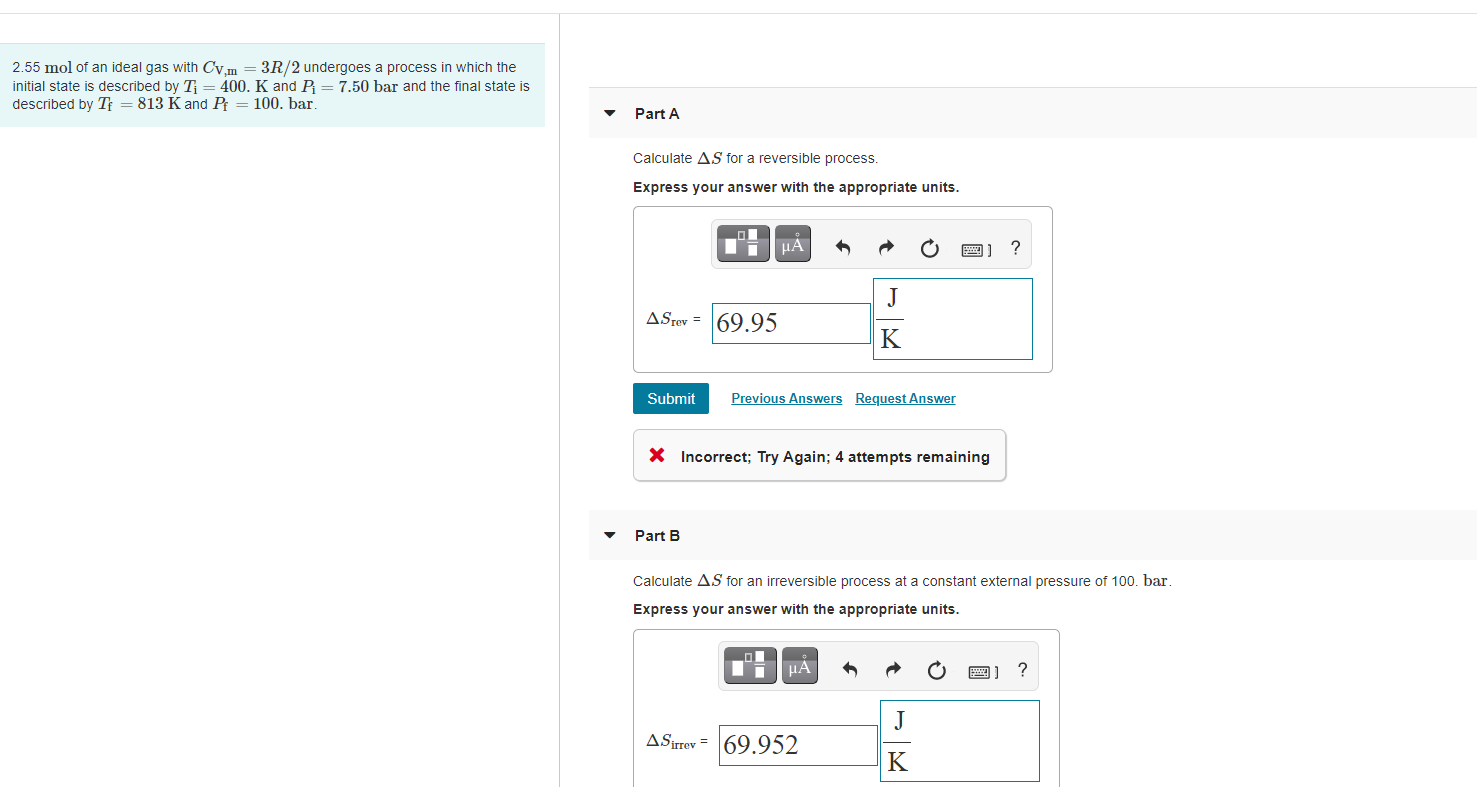
Solved 2.55 mol of an ideal gas with Cv,m = 3R/2 undergoes a

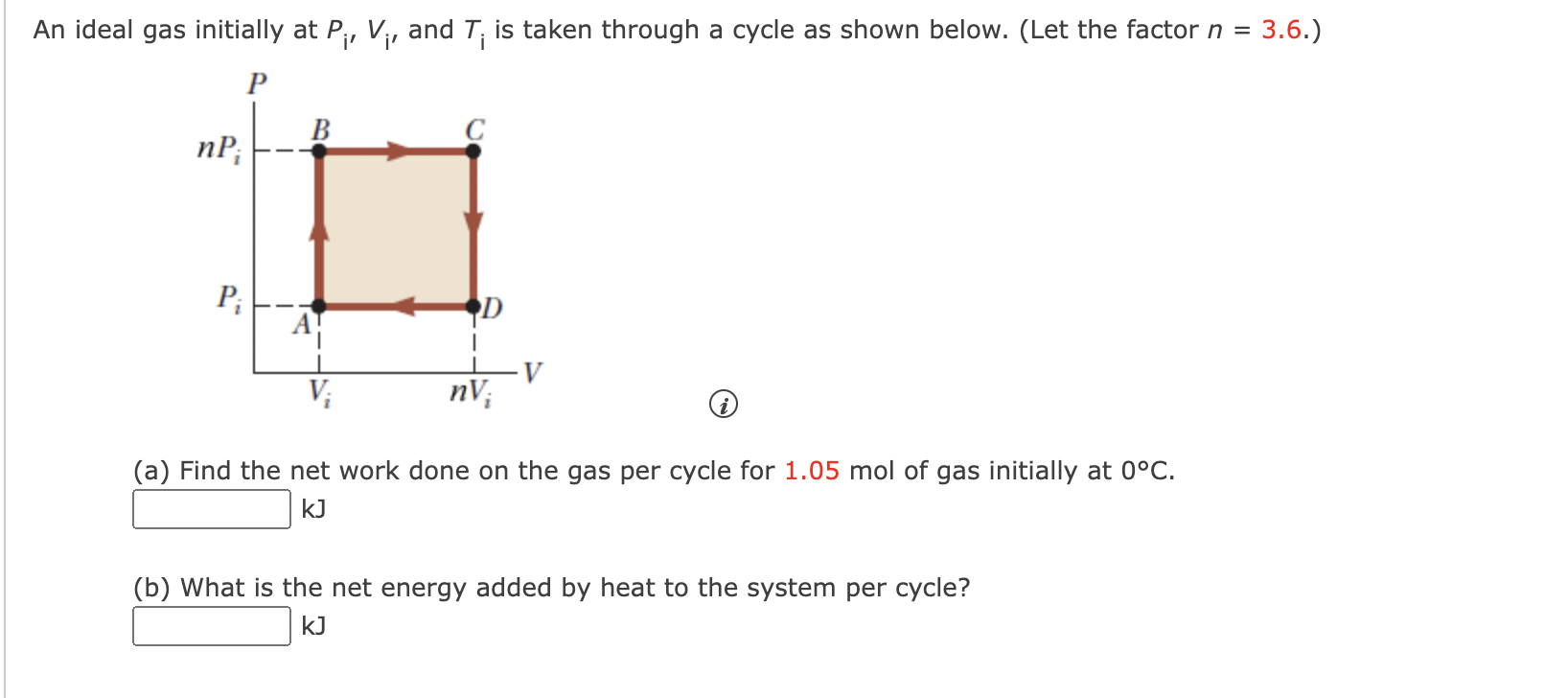
SOLVED: An ideal gas initially at Pi, V, and T;is taken through a cycle as shown in Figure What is the net energy added by heat to the gas per cycle for
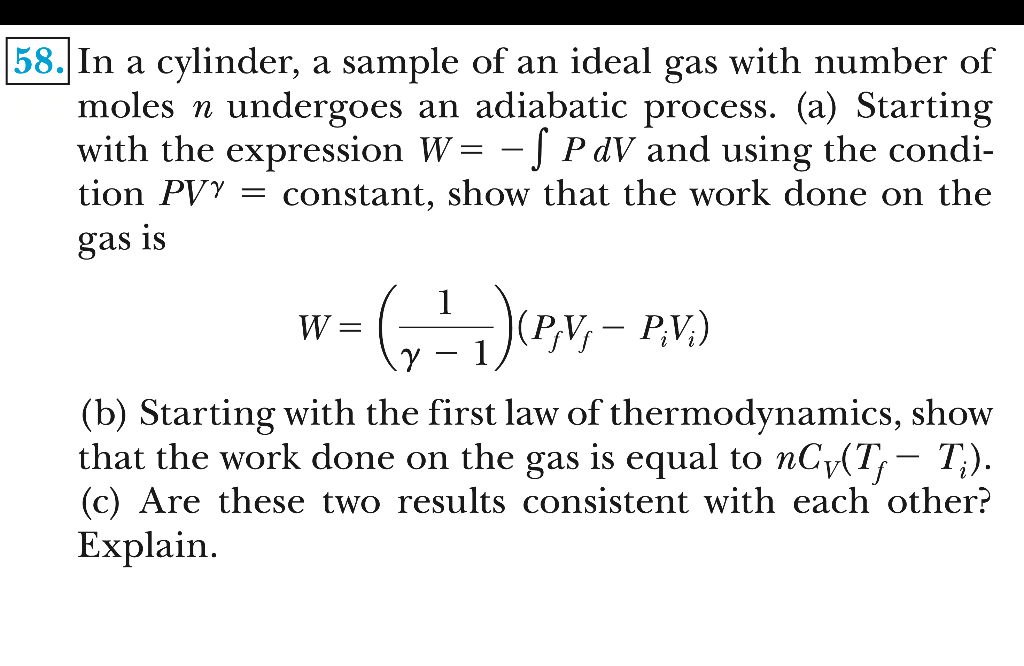
Solved In a cylinder, a sample of an ideal gas with number

An ideal gas initially P_i ,V_i , and T_i is taken through a cycle as shown in Figure. (a) Find the net work done on the gas per cycle 1.00 mol of
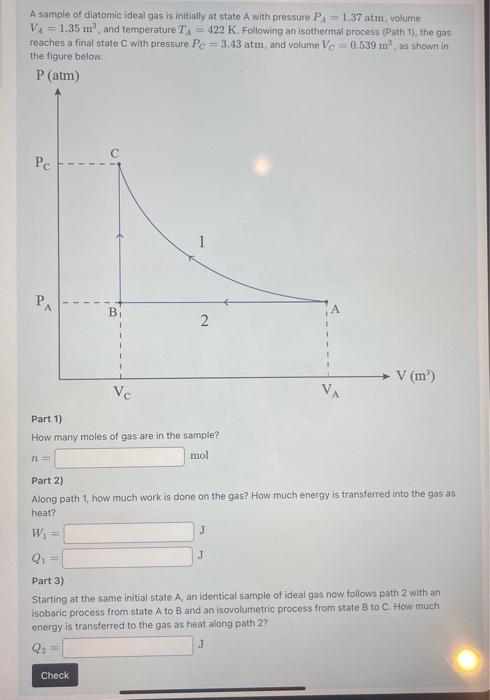
Solved A sample of diatomic ideal gas is initially at state
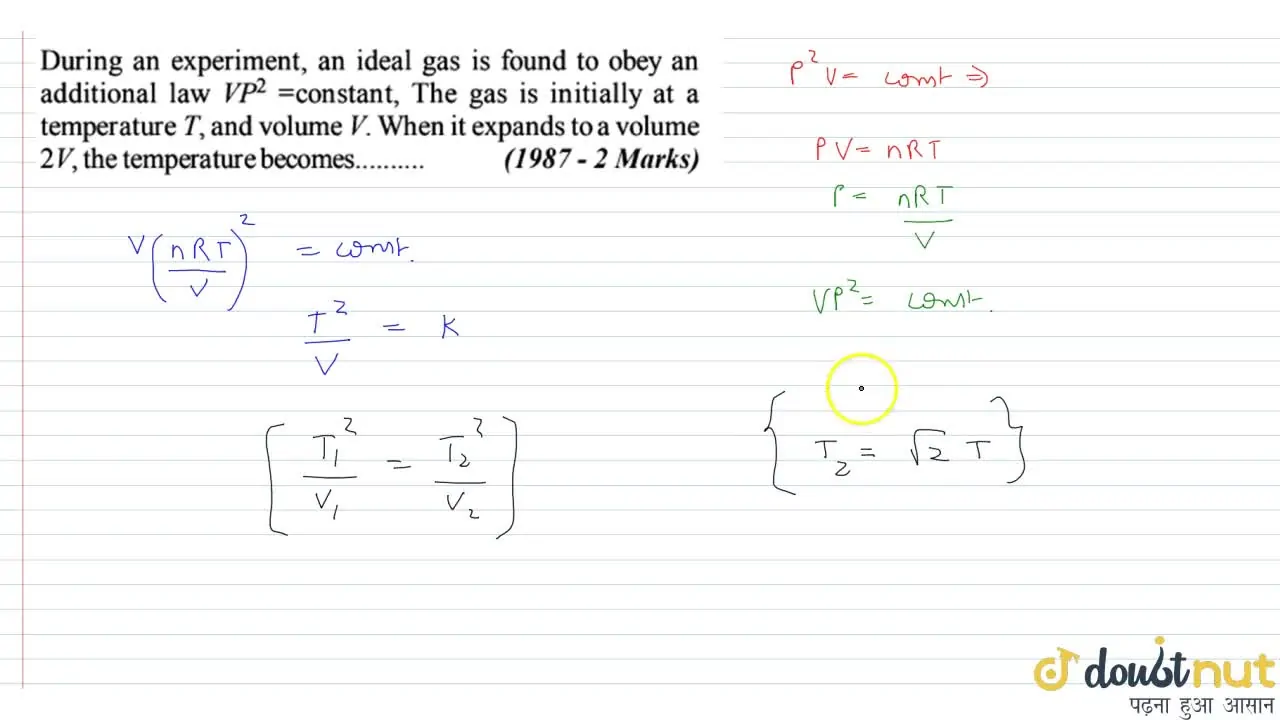
During an experiment, an ideal gas is found to obey an additional law
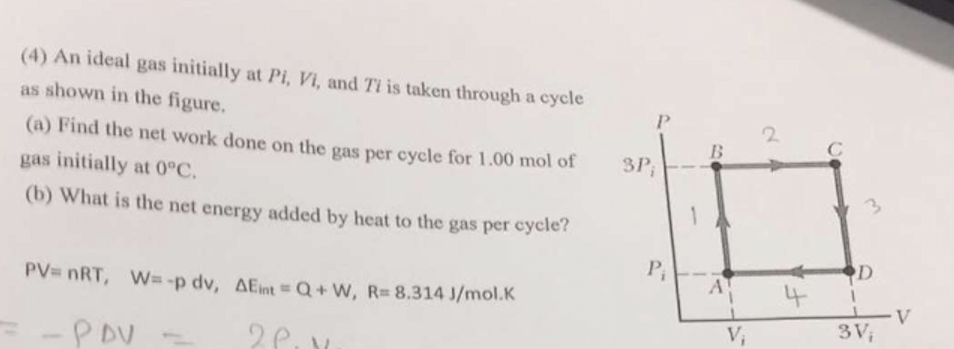
Solved (4) An ideal gas initially at Pi, Vi, and Ti is taken
The first law of thermodynamics
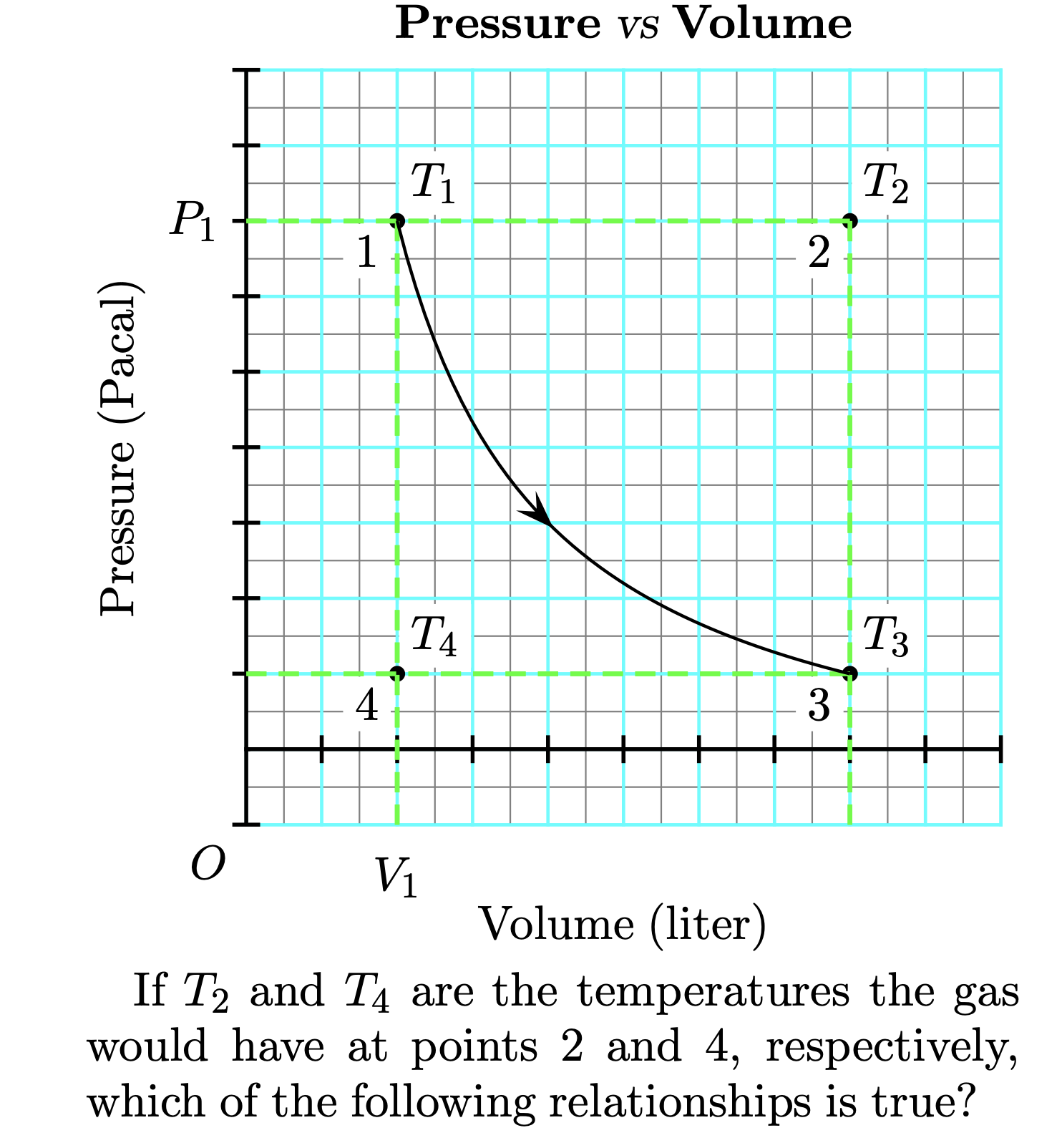
Solved An ideal gas is initially in a state that corre

Figure 20-29 showsa reversible cycle through which 1.00 mol of a monatomic ideal gas is taken. Volume V_{c}=8.00 V_{b} . Process b c is an adiabatic expansion, with p_{b} =10.0 mathrm{atm} and