At low pressure, the van der waal's equation is written as (P+ a/V
By A Mystery Man Writer
At low pressure, the van der waal's equation is written as (P+ a/V^2)V=RT . Then compressibility factor is then equal to :
At low pressure- the van der waal-s equation is written as -P- a-V-2-V-RT - Then compressibility factor is then equal to

At low pressure, Van der Waal's equation is reduced to [P+dfrac{a
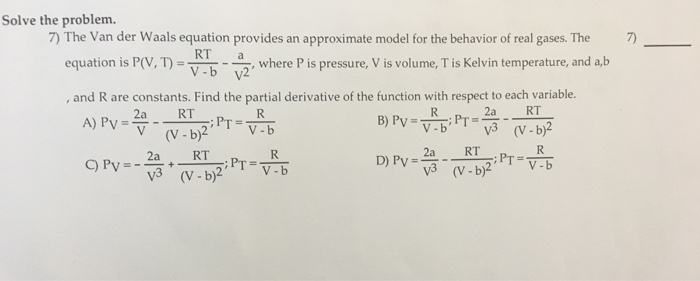
Solved The Van der Waals equation provides an approximate
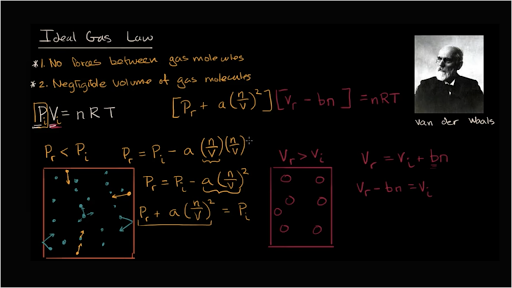
The van der Waals equation (video)

Vander-Waals Equation of State - GATE ME '15 S1
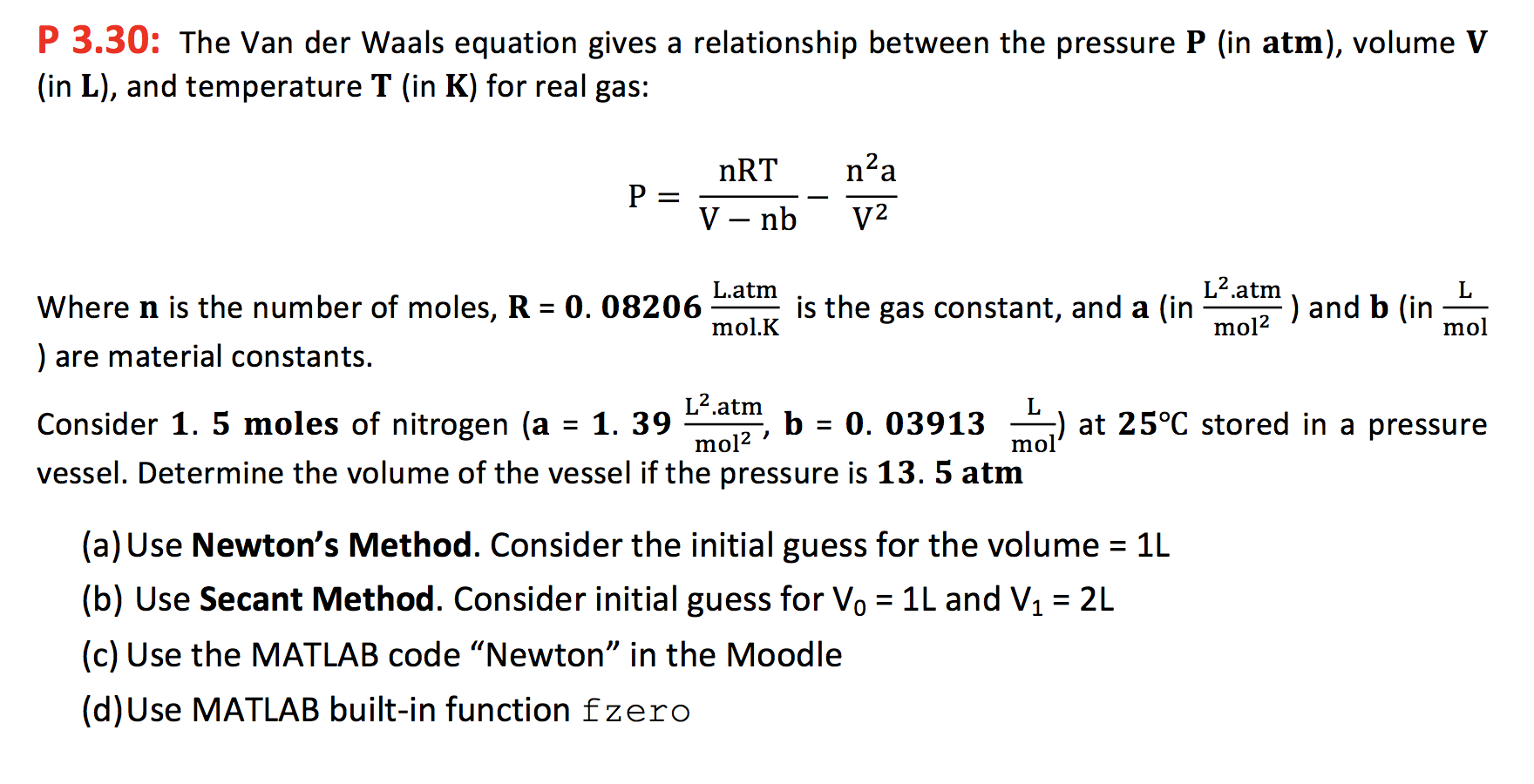
P 3.30: The Van der Waals equation gives a

At low pressure the van der Waals' equation is reduced to `[P +(a

A Quick Guide on Van der Waals Equation

At low pressures For 1 mole, the van der Waals equation is written

The van der Waals equation of the state is given as where, P

The van der Waals equation 1 mole of gas isleft(p+dfrac{a}{V^{2
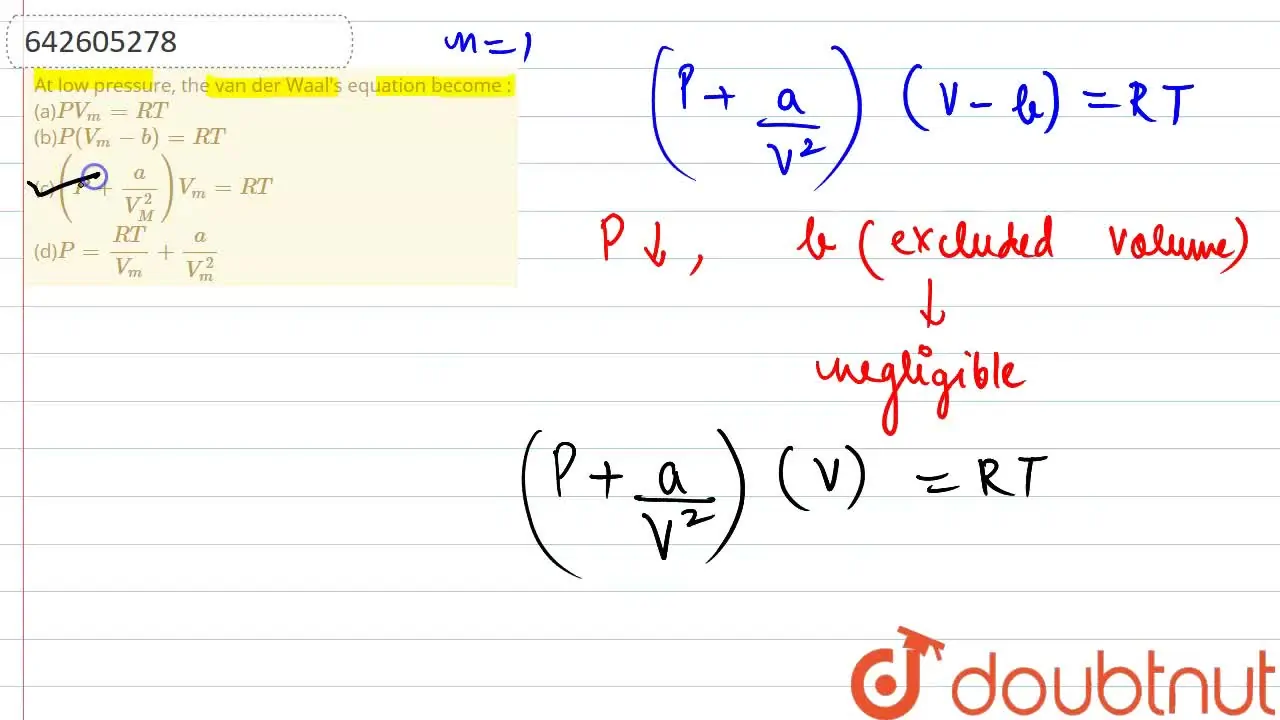
At low pressure, the van der Waal's equation become : (a)PV(m)=RT (b
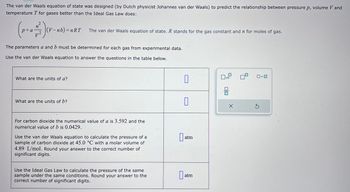
Answered: The van der Waals equation of state was…
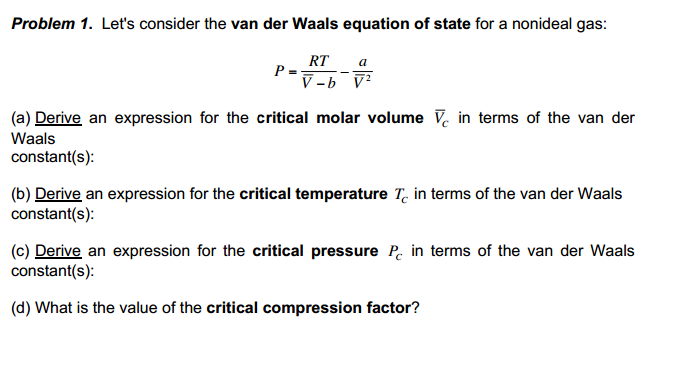
Solved Problem 1. Let's consider the van der Waals equation
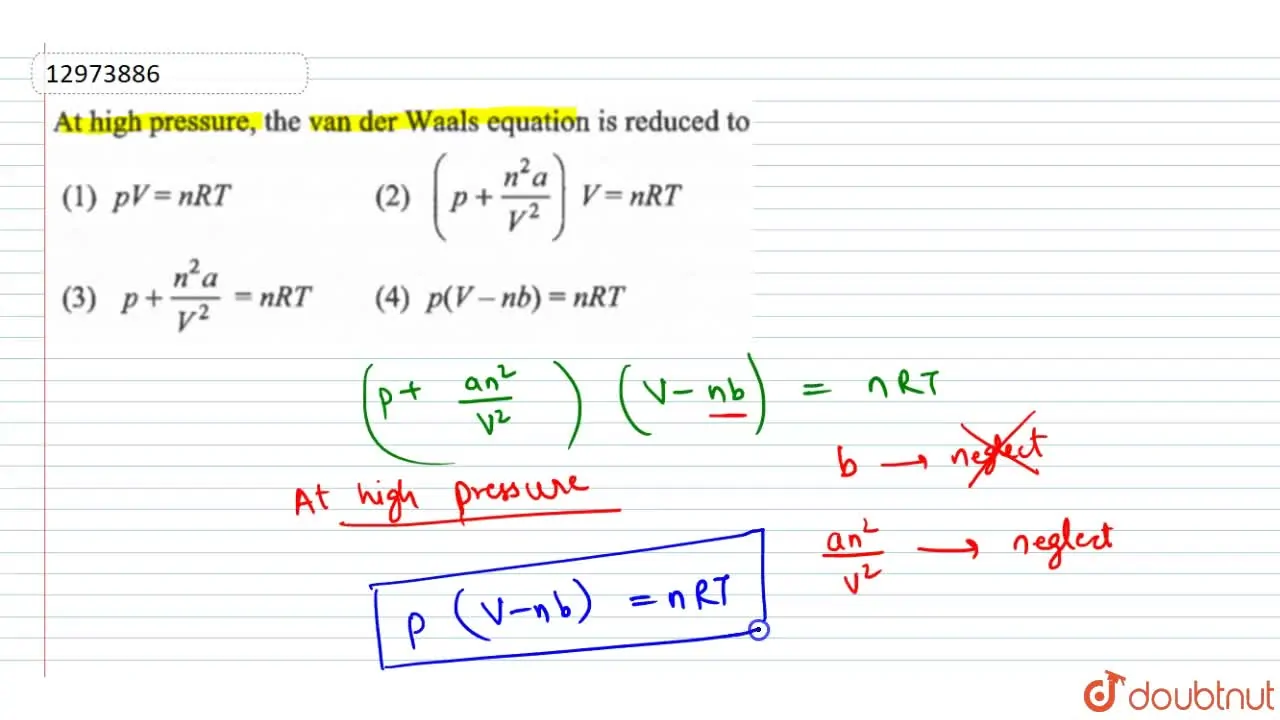
At high pressure , the van der Waals equation is reduced to
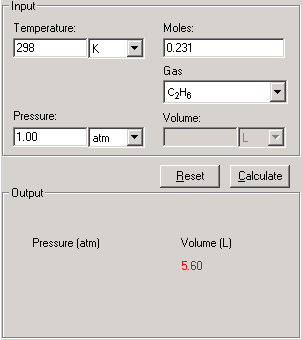
Real Gases - Van der Waals Equation